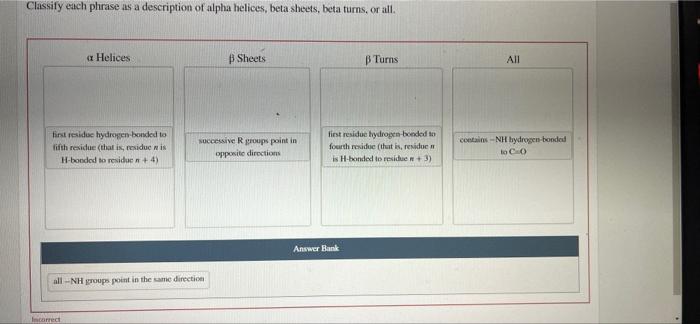
Classify each phrase as a description of alpha helices, unraveling the intricate structural tapestry of these ubiquitous protein building blocks. This discourse delves into the defining characteristics, functional roles, and computational analysis of alpha helices, providing a comprehensive understanding of their significance in the realm of protein science.
Alpha Helices: Structural Characteristics and Functional Roles: Classify Each Phrase As A Description Of Alpha Helices

Alpha helices are one of the most common secondary structures found in proteins. They are characterized by their helical shape and are stabilized by hydrogen bonds between the backbone NH and CO groups of adjacent amino acids. The typical length of an alpha helix is 10-15 amino acids, and the shape is described as a right-handed helix.
The hydrophobic side chains of the amino acids in an alpha helix are oriented towards the interior of the helix, while the hydrophilic side chains are oriented towards the exterior.Hydrogen bonding plays a critical role in the stability of alpha helices.
The hydrogen bonds between the backbone NH and CO groups of adjacent amino acids form a network of interactions that holds the helix together. The strength of these hydrogen bonds is influenced by the sequence of amino acids in the helix.
Amino acids with bulky side chains can disrupt the hydrogen bonding network and destabilize the helix.
Functional Roles of Alpha Helices, Classify each phrase as a description of alpha helices
Alpha helices play a variety of functional roles in proteins. They are often involved in protein-protein interactions, where they can mediate the binding of one protein to another. Alpha helices can also form transmembrane domains, which are regions of proteins that span the cell membrane.
These transmembrane domains allow proteins to interact with the extracellular environment. In addition, alpha helices can help to stabilize protein structures by providing a rigid framework.
Comparison to Other Secondary Structures
Alpha helices differ from other secondary structures in their structural features and stability. Beta sheets, another common secondary structure, are characterized by their pleated sheet-like shape. Beta sheets are stabilized by hydrogen bonds between the backbone NH and CO groups of amino acids in adjacent strands of the sheet.
Alpha helices are more stable than beta sheets, due to the presence of the hydrogen bonding network between adjacent amino acids in the helix.Random coils are another type of secondary structure that is found in proteins. Random coils are characterized by their lack of a regular structure.
They are more flexible than alpha helices and beta sheets, and they can adopt a variety of conformations.
FAQ Section
What is the typical length of an alpha helix?
Alpha helices typically range from 10 to 40 amino acids in length.
How do alpha helices contribute to protein stability?
Alpha helices stabilize proteins by forming hydrogen bonds between the backbone NH and CO groups of amino acids i and i+4, creating a helical structure.
What is the role of hydrophobic residues in alpha helices?
Hydrophobic residues cluster in the interior of alpha helices, away from the polar solvent environment, contributing to the stability of the helix.